Routine Practice Data Collection (Anwendungsbegleitende Datenerhebung, AbD)
Generating comparative evidence for rare diseases presents unique challenges due to the limited availability of study data. To address these gaps, Real-World Evidence (RWE) has gained increasing importance in the HTA process. Routine practice data collection is a key form of post-launch evidence generation required by the G-BA. Its aim is to reduce uncertainties from the initial benefit assessment and build a robust evidence base for reassessment and price negotiations.
With our extensive experience – including involvement in multiple ongoing AbD procedures – and a strong multidisciplinary approach, we provide comprehensive support and guidance throughout the entire AbD process.
Our STEP approach to successful AbD execution includes the following key services:
- Strategic Consulting
- Tailored Workshops
- Engagement during the Stakeholder Participation Process
- Preparation of Study Documents Including Study Protocols and SAPs
Our interdisciplinary teams specialize in biostatistics, medical writing, project management, and clinical operations, ensuring both methodological and operational excellence in the execution of your AbD study.
Dr. Stefanie Wüstner
Head of Business and Scientific Innovation
Stefanie is an HTA expert specializing in systematic literature reviews, comparative evidence generation, and strategic consulting at the intersection of clinical research, evidence synthesis, and regulatory requirements.
With an academic background in biosciences and public health and nearly ten years of experience in Health Technology Assessment (HTA), she supports pharmaceutical companies in the successful market positioning of their products - both within the German AMNOG framework and the European EU HTA process.
As Head of Business and Scientific Innovation, she is responsible for the professional advancement of interdisciplinary teams and drives practical, forward-thinking solutions - from automating complex processes and applying novel methodologies to integrating new technologies into everyday work.
She has extensive experience in the planning and execution of HTA dossiers, including the design of accompanying data collection strategies and the integration of real-world evidence (RWE) into assessment processes. Her ability to combine scientific depth with clear communication is reflected in numerous publications and international conference contributions.
Your service expert

Dr. Stefanie Wüstner
Head of Business and Scientific Innovation
Our services include
Setup
We provide expert guidance in the strategic planning of the AbD process, offering budget planning and consulting on stakeholder engagement.
Workshops
In tailored workshops, we support you in developing a clear course of action, conduct PICO scoping, and identifying potential evidence gaps through a structured analyze GAP-Analysis.
Engagement with G-BA and IQWiG
Our experienced consultants proactively guide you during the stakeholder participation process, drafting written responses to the IQWiG AbD concept and preparing you for expert hearings.
Study Documents
You benefit from the extensive experience of our HTA and Clinical Operations experts in planning, writing, and revision study documents and SAPs.
Methodological and Strategic Consulting
We share our experience and know-how in tackling the complex aspects of the AbD process, including calculation of patient numbers, systematic confounder identification, assessment of patient reported outcomes, and collaboration with registries.
AbD Implementation and Data Analysis
Our team supports you during and after the start of the routine practice data collection. We compile status reports, interim analyses, as well as final evaluations and interpretation.
AbD in Early Benefit Assessment
Since 2020, the G-BA can demand a post-launch routine practice data collection (Anwendungsbegleitende Datenerhebung, AbD) for new pharmaceutical products with certain approval pathways (conditional approval or approval under exceptional circumstances) or orphan drugs. The AbD process is initiated when available evidence for a benefit assessment is insufficient.

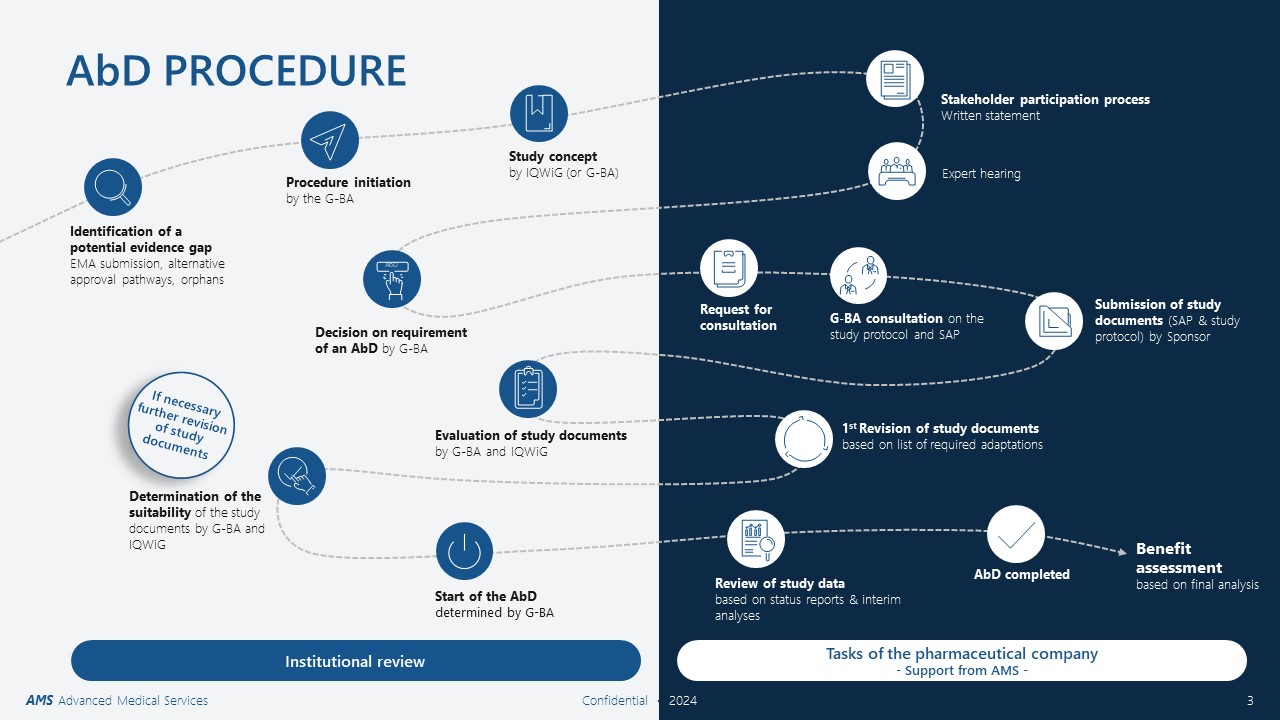
AbD Course of Action: When Does What Happen?
The AbD process is described in chapter 5 of the G-BA procedural rules and encompasses several steps. The development of the AbD concept by IQWiG and the written statement of the pharmaceutical company are essential steps in the process. If an AbD is requested, the pharmaceutical company is required to submit study documents (SAP and study protocol), which describe the methodology of the AbD. After the completion of the AbD, a new benefit assessment of the pharmaceutical product is conducted.
Early Consulting for Added Value Data Collection
Conference Abstracts
- Bogner K, Ebentheuer LM, Reiter B, Bierl M, Wüstner S (2024) Lessons Learned from Confounder Identification: Insights from German HTA Procedures, Value in Health, 27 (6), S273, https://doi.org/10.1016/10.1016/j.jval.2024.03.1506
-
Bierl M, Niederkofler D, Hogger S, Wüstner S (2023) Successful Use of Propensity Score Methods for HTA Germany: A Near-Impossible Task? Value in Health, 26 (12) Supplement S424, https://doi.org/10.1016/j.jval.2023.09.2216
-
Bogner K, Ebentheuer LM, Wegener M, Wüstner S (2023) Real World Evidence in German HTA: The Challenges of Comparative Routine Practice Data Collection (ABD) for Early Benefit Assessment (AMNOG) Value in Health, 26 (12), S386, https://doi.org/10.1016/j.jval.2023.09.2025
References







