European Health Technology Assessment (EU HTA) & National Dossiers
With the introduction of the EU HTA process, new requirements for the benefit assessment of pharmaceuticals and medical devices will come into effect. This unified European Assessment of new health technologies presents both opportunities and challenges.
Successfully navigating the EU HTA process requires proactive planning, flexibility, and a deep, comprehensive understanding of regulatory requirements.
AMS has been influencing the EU HTA landscape since 2015 through conferences, publications, EU HTA engagement, and EUCOPE membership. Our extensive hands-on experience and in-depth expertise – gained through the preparation and submission of rapid Relative Effectiveness Assessment (REA) Submission Files under EUnetHTA Joint Action 3 – ensure that you are thoroughly prepared and fully supported throughout the EU HTA process.
In addition, we have led projects focused on both structural and content-related preparation for EU HTA, including PICO anticipations, feasibility analyses for indirect treatment comparisons, and team training programs designed to optimize workflows and enhance interdisciplinary collaboration.
Leverage our expertise to strategically meet the requirements of the new EU HTA process and successfully implement them within your organization.
Dr. Jana Maurer
Deputy Director Medical Science
Jana began her career with a Ph.D. (Dr. rer. nat.) in basic research on chronic inflammatory bowel diseases at the Technical University of Munich. Since 2014, she has been a leading expert in strategic consulting and Medical Writing at AMS, with a particular focus on Health Technology Assessment (HTA), including EU HTA and AMNOG. There she provides strategic guidance to companies in developing product-specific market access strategies and supports the preparation of HTA dossiers for pharmaceuticals and medical devices in various indications, such as oncology and chronic inflammatory diseases. As a Senior Medical Writer, she leverages her expertise in writing and presentation to communicate scientific content with clarity and precision - ranging from HTA dossiers and peer-reviewed publications to oral and poster presentations. With over 20 years of experience in leading interdisciplinary teams, she has successfully managed the Medical Writing and EU HTA teams at AMS. From 2015 to 2017, she also headed the Market Access Group for medical devices, focusing on reimbursement strategies in Germany. Since 2022, as Deputy Director of the Medical Science department, she has been responsible for business & strategy development and client relations.
Our service expert

Dr. Jana Maurer
Deputy Director Medical Science
Our services include
EU HTA Training
Get ready for the EU HTA process – with our expert knowledge.
We strengthen your competence in the EU HTA process by sharing our expertise in a practical, tailored, and easy-to-understand way. Whether through customized workshops, hands-on presentations, or informative publications, we prepare you thoroughly for the EU HTA process. Benefit from our in-depth knowledge and navigate EU HTA challenges with confidence and strategic clarity.
Anticipating the Assessment Scope (PICO Anticipation)
A realistic timeline for your EU HTA dossier requires early anticipation of the EU HTA Coordination Group’s assessment scope. We support you in accurately predicting this scope – enabling proactive evidence generation and targeted dossier preparation.
Customized Playbook for the EU HTA Process
The EU HTA process presents new challenges for internal collaboration in companies – We assist you in establishing internal governance to ensure optimal preparation for new internal procedures.
Together, we develop a structured and practical playbook that aligns your key functions – Clinical Development, Regulatory Affairs, and Market Access – efficiently with the EU HTA requirements.
Development of a Tailored EU HTA Dossier – Scientifically and Strategically Sound
A compelling EU HTA dossier is critical for success in the Joint Clinical Assessment (JCA) and lays the groundwork for optimal pricing strategies at the national level. In close collaboration with you, we develop a scientifically sound and strategically refined dossier that effectively presents your evidence and meets all EU HTA regulatory requirements.
Evidence Generation for Your EU HTA Dossier - Scientific, Methodological & Strategic
Comparative evidence is crucial for addressing the diverse requirements of the assessment scope. Using systematic literature reviews (SLR) and study registry searches, we review the evidence landscape and identify relevant studies for your EU HTA dossier. Evidence synthesis and post hoc analyses are key success factors in the HTA process – and we are your trusted experts in delivering robust analyses.
Professional Support in Preparing Briefing Documents
Preparing briefing documents for European consultations – such as Joint Scientific Consultation (JSC), Parallel Consultation (EMA and local HTA authorities), or national consultations – is a crucial step in securing the success of your market access strategy. We offer pragmatic, targeted support in drafting these documents, ensuring that your key messages are clear and compelling. Rely on our expertise to make your consultation meetings successful and efficient.
Optimized Overall Process for EU HTA and National HTA (e.g., AMNOG Dossier)
With our extensive experience in the parallel preparation of European and national HTA dossiers for Germany, we offer an efficient and coordinated. We help you integrate different HTA processes, creating a synergistic overall strategy that saves time and optimally utilizes resources.
Transition from National to EU HTA: When Will What Be Switched?
Since January 2025, the regulation on the EU HTA has been implemented. As a result, the clinical domains of an HTA for newly approved pharmaceuticals will be conducted at the European level rather than at the national level – this is the EU HTA. For different medicinal products and medical devices, health technology assessments at EU level (Joint Clinical Assessment, JCA) will become mandatory at different points in time. By 2030, JCAs will be compulsory for all medicinal products.
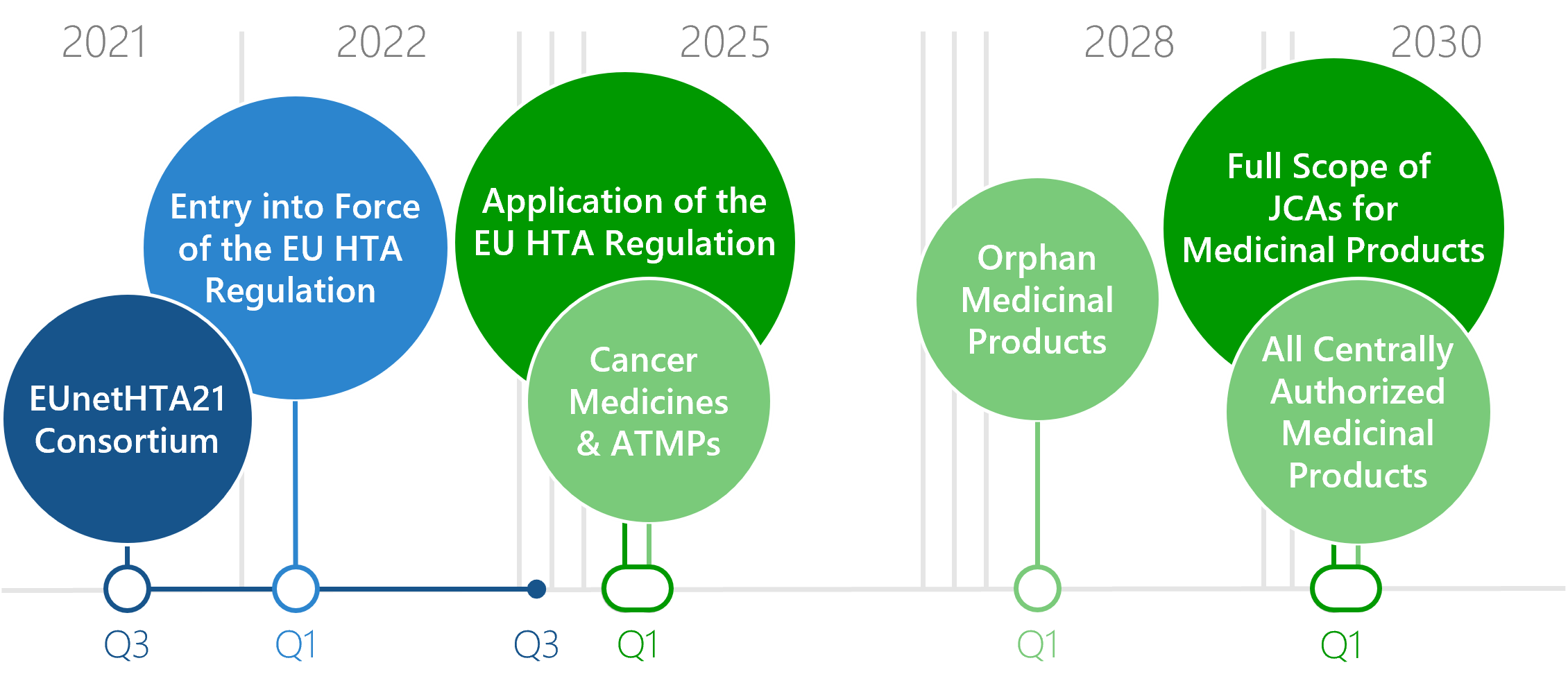
Download: Flyer Timelines Inkrafttreten des EU HTA
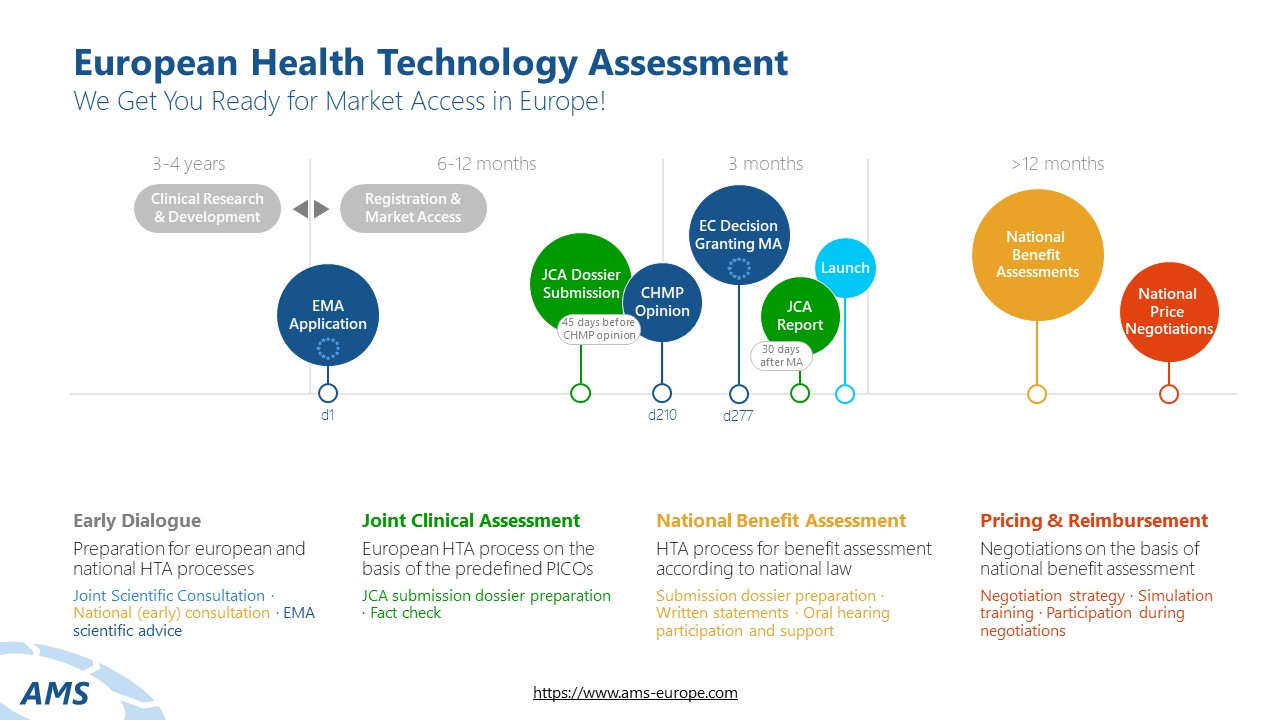
The Joint Clinical Assessment (JCA) Process
The JCA is a central component of the EU HTA process, standardizing the clinical evaluation of health technologies at the European level. It is overseen by the HTA Coordination Group of EU Member States. The JCA dossier includes clinical data on relative efficacy and safety and must be submitted no later than 45 days before the CHMP Opinion.
After a thorough review of the submitted information by the assessors, the JCA report is published no later than 30 days after EMA market authorization and made available to national HTA authorities.
PICO Scoping for the Assessment Scope
Before submitting the JCA dossier, a scoping process is conducted within the EU HTA Coordination Group. The results of this process define the research question and the assessment scope for the dossier. The scoping process aims to establish the PICO criteria (Population, Intervention, Comparator, Outcomes), which set the framework for the assessment and define the data requirements for the manufacturer.
JCA Dossier Preparation
Once the assessment scope is finalized, health technology developers typically have only 100 days to prepare the dossier. The JCA dossier includes a systematic evidence review and addresses the key questions defined in the assessment scope regarding relative efficacy and safety.
As the primary evidence base for the JCA report, the dossier contains all relevant clinical studies – including direct and indirect comparisons – ensuring a scientifically sound benefit assessment.
Evidence Generation for Your EU HTA Dossier – Scientific, Methodological & Strategic
Comparative evidence is crucial for addressing the diverse requirements of the assessment scope. Using systematic literature reviews (SLR) and study registry searches, we review the evidence landscape and identify relevant studies for your EU HTA dossier. Evidence synthesis and post hoc analyses are key success factors in the HTA process – and we are your trusted experts in delivering robust analyses.
Whether it’s systematic study searches, endpoint selection, surrogate validation, subgroup or sensitivity analyses, network meta-analyses, or indirect comparisons – we provide comprehensive guidance on HTA requirements. Together, we develop a tailored data strategy that aligns with your scientific, regulatory, and economic goals.
Additionally, we offer complementary analyses based on primary datasets (ADaM) and various evidence syntheses – providing consulting, calculations, and interpretations all in one place. Through targeted visualization techniques, we make complex data tangible, creating a solid foundation for HTA decision-making processes.
Leverage our expertise for reliable results and successful HTA strategies.

Original Articles in Peer-reviewed Journals
- Eberle, K.; Hagemann, L.-M.; Schweitzer, M.K.; Justl, M.; Maurer, J.; Carls, A.; Reuter, E.-M. The PICO Puzzle: Can Public Data Predict EU HTA Expectations for All EU Countries? J. Mark. Access Health Policy 2025, 13, 32. https://doi.org/10.3390/jmahp13030032
- Schweitzer MK, Dold MN, Genet A, Gossens K, Klein-Hessling T, Löffler N, Rabel M, Rasch A, Reuter EM, Schmelcher J, Wolfram N, Werner S (2023). Shaping a suitable EU HTA dossier template: why the German template is not fit for purpose. Eur J Health Econ. 2023 Oct 16. doi: 10.1007/s10198-023-01631-5
- Schweitzer MK, Dold MN, Genet A, Gossens K, Klein-Hessling T, Löffler N, Rabel M, Rasch A, Schmelcher J, Werner S, Wolfram N (2023). Auswirkungen der neuen Dossieranforderungen Monitor Versorgungsforschung 03/23, p26–30, doi: http://doi.org/10.24945/MVF.03.23.1866-0533.2500
- Genet A, Bogner K, Goertz R, Böhme S, Leverkus F (2023). Safety analysis of new medications in clinical trials: A simulation study to assess the differences between cause-specific and subdistribution frameworks in the presence of competing events. Research Square. doi: 10.21203/rs.3.rs-2475247/v1
- Kisser A, Knieriemen J, Fasan A, Eberle K, Hogger S, Werner S, Taube T, Rasch A (2022). Towards compatibility of EUnetHTA JCA methodology and German HTA: a systematic comparison and recommendations from an industry perspective. Eur J Health Econ 23(5):863-878, doi: 10.1007/s10198-021-01400-2
- Wüstner S, Hogger S, Gartner-Freyer D, Lebioda A, Schley K, Leverkus F (2022). Clinical Evidence Informing Treatment Guidelines on Repurposed Drugs for Hospitalized Patients During the Early COVID-19 Pandemic: Corticosteroids, Anticoagulants, (Hydroxy)chloroquine. Front Public Health 18;10:804404, doi: 10.3389/fpubh.2022.804404
Further Publications (Workshops/ Webinars/ Presentations/ Conference Abstracts)
- Reuter, E., Stein, L., Rittmeyer, C., Wüstner, S., Werner, S. (2025). More Similarities than Differences Methodological Approaches for Evidence Synthesis in EU and German HTA. Accepted for DIA Europe 2025. doi:26226/m.6756a9c308a81a4962784387
- Reuter E. Klein-Hessling T, Rittmeyer C, Schweitzer M.K., Werner S. (2024). PD149 Comparison Of The Draft European Union Health Technology Assessment Template With Germany’s AMNOG Template. International Journal of Technology Assessment in Health Care.;40(S1):S151-S151. doi:10.1017/S0266462324003829
- AMS (Reuter, E., Rua del Barrio, M.) (2025): EU HTA- Introduction to the new EU HTAR & Spanish HTA. BIOVAL Clúster BIO Comunidad Valenciana
- AMS (Reuter, E., Eberle K.) (2024): Auswirkungen der EU-HTA-Verordnung auf die Gesundheitsindustrie. BIOPRO Baden-Württemberg GmbH
- AMS (Eberle K), EUCOPE (2022) European HTA: What have we learned, what lies ahead?, Webinar, Information on EUCOPE website
- AMS & Vfa (2021) New requirements for AMNOG-dossiers: Investigation of considered evaluations in the context of the benefit assessment by IQWiG and G-BA, Report, Information on Vfa website
- Panni, T., Thiele, A., Carls A., Wallstab, A., Eberle, K. & Schleibner S (2018) PHP281 – THE METHODOLOGICAL DIFFERENCES OF EUROPEAN JOINT ASSESSMENT AND GERMAN HTA: AN EMPIRICAL APPROACH. Value in Health 21, p197-S8. https://doi.org/10.1016/j.jval.2018.09.1175
- Rüther A, Herrmann K, Hebborn A, Perleth M, Schwarzer R, Schürmann C, et al. HTA und aktuelle Herausforderungen: Harmonisierung, Real World Data und Surrogatparameter. HTA – How to tackle pressing challenges: International Harmonization, Real World Data, and Surrogates. GMS Medizinische Informatik, Biometrie und Epidemiologie. 2018;Vol. 14(1). https://dx.doi.org/10.3205/mibe000180
- Panni, T., Eberle, K., Seiler, N., Brüderl, M., Burkert-Kautzsch C, Carls A, et al. (2017) VP181 From National To European Assessment – The German Case. International Journal of Technology Assessment in Health Care, 33(S1), 234-5, https://doi.org/10.1017/S0266462317004147
- Hemmerling J, Eberle, K., Hogger S, Gupta M, Ullraum, A, & Seemüller S (2017) PP096 European Union-Health Technology Assessments For Medical Devices – How To Overcome Reimbursement Divergence. International Journal of Technology Assessment in Health Care, 33(S1), 116-117. https://doi.org/10.1017/S0266462317002616
- Gerlinde Jänel. Europäisches und nationales HTA – gleiches Ziel, gleiche Methodik? European and National – same aim, same method? HEC 2016: Health – Exploring Complexity. Joint Conference of GMDS, DGEpi, IEA-EEF, EFMI. München, 28.08.-02.09.2016. Düsseldorf: German Medical Science GMS Publishing House; 2016. DocAbstr. 876-2 https://dx.doi.org/10.3205/16gmds100
References







