HTA Statistics
Generating, Analyzing, and Communicating Evidence – Strategically and Effectively
We provide comprehensive consulting and support on all aspects of evidence generation, statistical analysis, and results presentation for EU and national HTA. Our goal is to strategically align with HTA requirements while shaping your evidence base to achieve the best possible outcomes.
To ensure your evidence is impactful, we apply a broad range of advanced statistical methods tailored to your specific needs.
With a team of over 24 biostatisticians, we guide you through the entire HTA process – from feasibility analysis to the compelling presentation of your data.
Silke Seemüller
Director Biostatistics
Silke has over 20 years of experience in biostatistics. After completing her degree in statistics in 2004 at Ludwig Maximilian University of Munich, she began her career at the Technical University of Munich, focusing on multiple sclerosis research with an emphasis on data management and statistics.
Since 2010, Silke has been working at AMS - initially as a SAS programmer, later as a senior biostatistician, and from 2017 as an Associate Director of Biostatistics. In November 2024, she took over as Director of Biostatistics.
Her responsibilities at AMS include not only client consulting but also the planning, execution, analysis, and reporting of clinical studies from Phase I to IV, as well as non-interventional studies. Since the implementation of the German Pharmaceutical Market Restructuring Act (AMNOG), she has played a key role in the preparation of benefit assessments. She is responsible for the statistical aspects of benefit dossiers and provides strategic consulting to clients.
Your service expert

Silke Seemüller
Director Biostatistics
Our services include
Evidence Strategy Consulting
We help you define the optimal evidence strategy based on HTA requirements and assessment practices of HTA bodies. We identify potential evidence gaps and develop tailored solutions to strengthen your data foundation. In close collaboration with you, we design statistical analysis plans (SAP/data requests) to establish a robust and compelling evidence base.
HTA-Specific Complementary Analyses
For your studies, we perform all necessary complementary statistical analyses – such as subgroup analyses or additional data cuts based on patient-level data – in compliance with the CDISC ADaM standard and aligned with the current HTA requirements.
We also design and conduct feasibility assessments and simulation studies to address specific research questions in a targeted, efficient manner.
In addition, we support you in effectively communicating key insights within your organization, to regulatory authorities, and to other stakeholders.
Evidence Synthesis and advanced Statistical Analyses
To demonstrate the value of your medicinal product, we apply a wide range of advanced statistical methods tailored to your data context and strategic objectives of your analysis. Based on the available evidence and the assessment goals, we identify the appropriate methodologies perform the analyses, and present the results in a clear, structured, and decision-oriented format.
Data Workshop with Visual Dossier Simulation
A convincing HTA dossier starts with a clear and coherent data strategy. In our interactive data workshop, we collaborate with you to develop a comprehensive strategy for your HTA submission.
AMNOG Benefit Assessment
Since pioneering in 2010, AMS has become one of the market leaders in AMNOG benefit assessments – supporting over 300 dossier projects across more than 50 indications. Our clients value our comprehensive and in-depth statistical expertise.
European HTA
Since 2015, AMS has been actively involved in EU HTA. We support you in developing a fit-for-purpose evidence package for the EU HTA by aligning your data strategy with the specific requirements of the Joint Clinical Assessment (JCA) and by fully addressing the diversity of relevant PICO criteria. Learn more about our expertise and services related to EU HTA here.
Routine Practice Data Collection (Anwendungsbegleitende Datenerhebung, AbD)
With our extensive experience in both clinical research and HTA, we are ideally positioned to support you in the study planning and statistical analysis of non-randomized comparisons within the framework of AbD procedures.
Evidence Synthesis & Advanced Statistical Analyses
To demonstrate the value of your medicinal product, we apply a wide range of advanced statistical methods tailored to your data context and strategic objectives of your analysis. Based on the available evidence and the assessment goals, we identify the appropriate methodologies perform the analyses, and present the results in a clear, structured, and decision-oriented format.
Our expertise includes both direct and indirect treatment comparisons – such as meta-analyses, network meta-analyses, Bucher ITC, MAIC, STC, and ML-NMR – enabling indirect comparisons in the absence of head-to-head trials. To address potential confounding, we apply propensity score methods and covariate adjustments. Clinically relevant effect modifiers are accounted for through survival analyses and multivariable regression models.
Where appropriate, we incorporate simulation studies and multiple imputation techniques to quantify uncertainty and support sensitivity scenarios.
Our goal is to deliver a robust and credible evidence base that supports precise conclusions on efficacy, safety, and quality of life – in line with HTA standards.

Data Workshop with Visual Dossiers Simulation
A convincing HTA dossier starts with a clear and coherent data strategy. In our interactive data workshop, we collaborate with you to develop a comprehensive strategy for your HTA submission.
Using our visual dossier simulation, we translate your analyses into intuitive visualizations and explore different scenarios. This approach enables us to jointly identify the most effective strategy for your benefit assessment – based on transparency, consistency, and strategic focus.
Data Workshop with Visual Dossiers Simulation
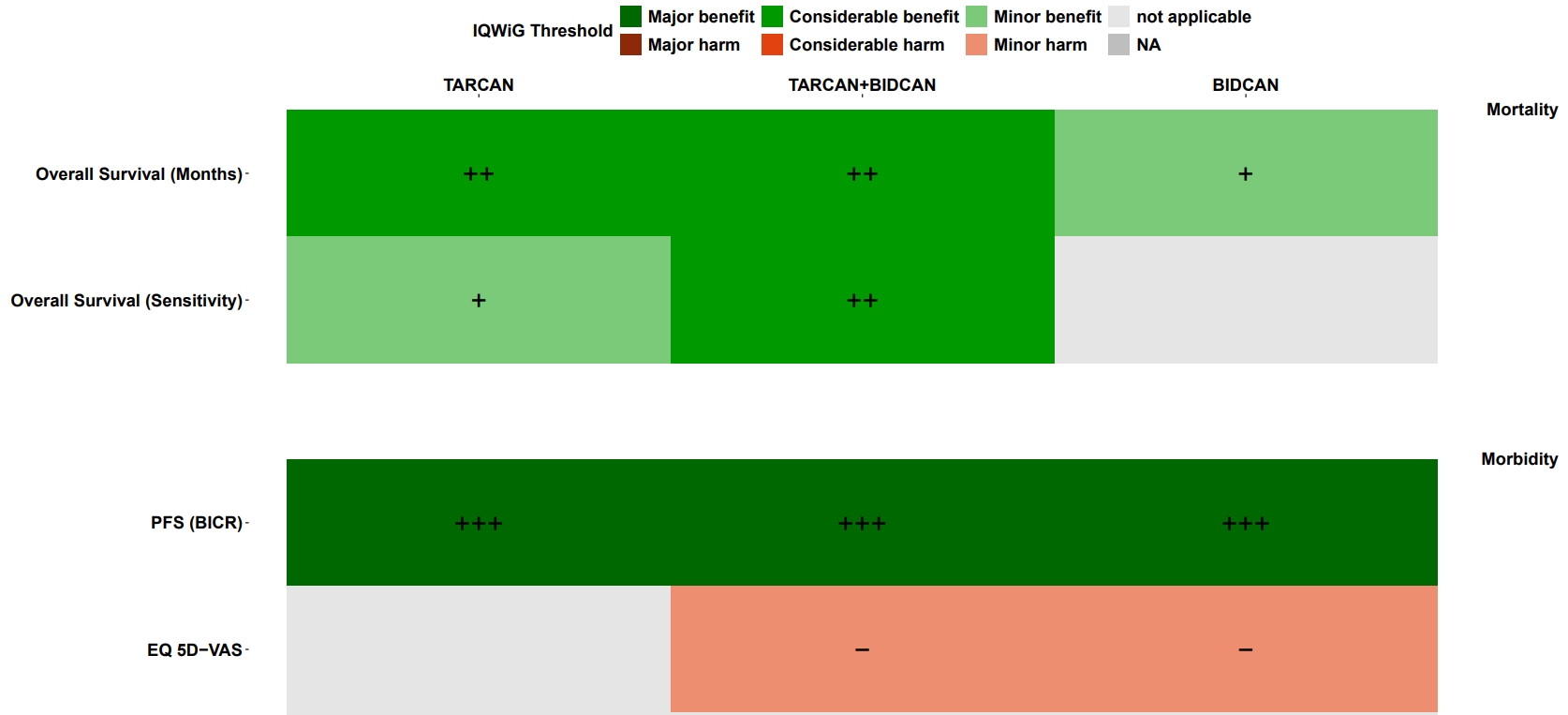
AMNOG Benefit Assessment
Since pioneering in 2010, AMS has become one of the market leaders in AMNOG benefit assessments – supporting over 300 dossier projects across more than 50 indications. Our clients value our comprehensive and in-depth statistical expertise.
With us, you receive everything from a single source: from strategic planning and data analysis to the clear, impactful presentation of results in your HTA dossier.
In addition, we provide in-depth consulting to support your evidence-based strategy and strengthen your position in benefit assessments.

European HTA (EU HTA)

Since 2015, AMS has been actively involved in EU HTA. We support you in developing a fit-for-purpose evidence package for the EU HTA by aligning your data strategy with the specific requirements of the Joint Clinical Assessment (JCA) and by fully addressing the diversity of relevant PICO criteria. Learn more about our expertise and services related to EU HTA here.
Routine Practice Data Collection (Anwendungsbegleitende Datenerhebung, AbD)
With our extensive experience in both clinical research and HTA, we are ideally positioned to support you in the study planning and statistical analysis of non-randomized comparisons within the framework of AbD procedures.
From initiating the process, through the development of the study protocol and statistical analysis plan (SAP), to study conduct, interim analyses, and final evaluation – we guide you through the entire process.
Learn more about our expertise and services related to AbD here.
Publications
- Genet, A., Bogner, K., Goertz, R. et al. Safety analysis of new medications in clinical trials: a simulation study to assess the differences between cause-specific and subdistribution frameworks in the presence of competing events. BMC Med Res Methodol 23, 168 (2023). https://doi.org/10.1186/s12874-023-01985-7
- Gillhaus, Johanna & Goertz, Ralf & Jeratsch, Ulli & Leverkus, Friedhelm. (2017). Surrogatvalidierung durch Korrelation und Surrogate Threshold Effect – Ergebnisse von Simulationsstudien Validation of surrogates by correlation and surrogate threshold effect – Results of simulation studies. GMS Medizinische Informatik, Biometrie und Epidemiologie 2017, Vol. 13(1), ISSN 1860-9171. 13. 12. https://doi.org/10.3205/mibe000168.
- S. Wüstner, S. Hogger, D. Gartner-Freyer, A. Lebioda, K. Schley, F. Leverkus (2022): Clinical Evidence Informing Treatment Guidelines on Repurposed Drugs for Hospitalized Patients During the Early COVID-19 Pandemic: Corticosteroids, Anticoagulants, (Hydroxy)chloroquine, Front Public Health, https://doi.org/10.3389/fpubh.2022.804404
- A. Kisser, J. Knieriemen, A. Fasan, K. Eberle, S. Hogger, S. Werner, T. Taube, A. Rasch (2021): Towards compatibility of EUnetHTA JCA methodology and German HTA: a systematic comparison and recommendations from an industry perspective, Eur J Health Econ, https://doi.org/10.1007/s10198-021-01400-2
Conference abstracts
Bierl M., Niederkofler D., Hogger S., Wüstner S. (2023) Successful Use of Propensity Score Methods for HTA Germany: A Near-Impossible Task? Value in Health, 26 (12) Supplement S424, https://doi.org/10.1016/j.jval.2023.09.2216
References







