Medical Devices – Post-Market Clinical Follow-up (PMCF)
Clinical Evaluation is an ongoing process, and compliance with the Medical Device Regulation 2017/745 mandates the proactive gathering of clinical data for CE marked products. The objective is to continually validate safety and performance throughout the product’s lifecycle, while also identifying and mitigating emerging risks.
AMS specializes in conducting Post-Market Clinical Follow-up (PMCF) studies in alignment with national legislations, good clinical practice standards, and requests by notified bodies. Allow us to assist you in executing a comprehensive PMCF study for your Medical Device, ensuring ongoing compliance and enhanced patient safety. Trust AMS to safeguard the effectiveness and reliability of your medical innovations through post-market clinical assessments.
Dr. Gabriele Feldmann
Clinical Operations Manager
With a robust scientific foundation in pathophysiology and pathobiochemistry, Gabriele has been a dedicated professional in clinical research for over 30 years. Her extensive expertise spans site selection, monitoring, and project management across international studies covering all phases of development. Gabriele specializes in medical device investigations, overseeing studies involving devices across all risk classes.
Gabriele has established and nurtured a team of AMS experts, focusing on this area of clinical research. She provides tailored consultancy to small and medium-sized sponsors, guiding them through the planning and execution of pivotal studies and Post-Market Clinical Follow-up (PMCF) investigations. Her strategic insights and hands-on experience ensure the success of complex medical device studies.
Gabriele’s commitment to advancing clinical research through innovative solutions and collaboration makes her an invaluable asset to AMS and its mission to improve healthcare outcomes.
Your service expert

Dr. Gabriele Feldmann
Clinical Operations Manager
Our services include
Strategic Consulting
Dedicated Teams for Targeted Indications: Embedded in the core philosophy of AMS is the establishment of specialized teams focused on enhancing therapeutic expertise across all facets of our Clinical Research services.
Feasibility
Recognizing its paramount importance, AMS places significant emphasis on conducting a comprehensive feasibility analysis across various levels prior to finalizing the Clinical Trial Protocol.
Medical Writing
Our dedicated team specializes in providing unparalleled Medical Writing services that are not only scientifically sound but also tailored to meet the unique needs of our clients and the applicable regulatory requirements.
Project Management
The role of the Project Manager is key in ensuring the success of any Clinical Study. Throughout your project, our Project Managers at AMS will proactively engage with you and your team, offering innovative solutions to overcome project challenges.
Regulatory Affairs
Regulatory requirements for drug development globally are becoming more complex – the AMS Regulatory Affairs team, together with our other Services, has the expertise to navigate you through these when planning your drug development strategy.
Data Management
AMS follows the CDISC data standards, for the collection of data according to CDASH and for analysis and submission of data according to ADaM and SDTM.
AMS ePRO®
You can now capture your patients data Anytime Anywhere with AMS-ePRO® to provide you with more clinically relevant data to help your trial move.
Monitoring/ Risk-based Monitoring/ Remote and Centralized Monitoring
AMS stands out as a leader in clinical research, boasting a team of dedicated Clinical Research Associates (CRAs) employed across Europe and expanded by global alliance partners. Our success is consistently validated by the positive feedback from our clients.
Clinical Safety and Pharmacovigilance
AMS Clinical Safety and Pharmacovigilance experts, with diverse backgrounds in medical, pharmaceutical and natural sciences, support you throughout the entire drug development journey – from the initial stages to authorization and post-marketing.
Biostatistics
All services required in the lifecycle of clinical development projects, beginning with biostatistical consulting during development of the project concept up to contributions for submissions or HTA dossiers and post approval studies.
AMS Clinical Research Consulting & Specialized Indication Expertise
Dedicated Teams for Targeted Indications: Embedded in the core philosophy of AMS is the establishment of specialized teams focused on enhancing therapeutic expertise across all facets of our Clinical Research services.
Our Clinical Research team at AMS comprises experienced physicians, certified medical writers, and skilled biostatisticians, providing unwavering support to our clients throughout the study lifecycle. This support includes, but is not limited to:
- Clinical Trial Design
- Protocol Setup
- Site and Patient Selection
- Medical Review
- Medical Input to Final Report
During the crucial early planning phase of your Clinical Study, AMS specialists contribute significant input to protocol design. This early planning stage can profoundly influence the subsequent success of your study and the achievement of your development targets.

Engage with our specialists at AMS to tap into their expertise. AMS Consulting stands as an independent service, accessible at the preliminary stages of your trials, even before committing to the full outsourcing process. Benefit from our specialized insight to optimize your study’s design and optimize the likelihood of achieving your development objectives.
Clinical Research Indication Experience:
- Immunology
- Oncology/ Hematology
- Neurology
- Rheumatology
- CNS
- Endocrinology
- Cardiology
- Respiratory
- Nephrology
- Gastrointestinal
- Infectious Disease/ Vaccines
- Dermatology
- Ophthalmology
- Pediatrics
- Rare Diseases
Feasibility

Recognizing its paramount importance, AMS places significant emphasis on conducting a comprehensive feasibility analysis across various levels prior to finalizing the Clinical Trial Protocol.
At the protocol level, our feasibility assessment ensures that the Clinical Study concept aligns with critical criteria, meeting global and local medical standards, Competent Authority requirements, and ethical principles.
Operating at the country level, our feasibility analysis guarantees protocol compliance with local or regional requirements – a crucial consideration in the context of global trial designs.
Site-level feasibility evaluations provide robust insights into the capacity of target sites to meet protocol requirements and patient selection criteria. A well-executed feasibility study supports the development of robust project budgets and timelines, contributing to predictable patient recruitment.
AMS offers a variety of distinctive feasibility techniques seamlessly integrated into the clinical system landscape of the project, including direct links to Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC), thus enhancing overall workflow efficiency.
Engage with our feasibility experts early in your Clinical Study planning phase to leverage our support for your project design.
AMS feasibility is a standalone service, allowing you to contract us before the formal outsourcing of your trial conduct begins. Take advantage of our expertise to enhance the success of your clinical trials.
Medical Writing in Clinical Research
Our dedicated team specializes in providing unparalleled Medical Writing services that are not only scientifically sound but also tailored to meet the unique needs of our clients and the applicable regulatory requirements. Our adept medical writers possess a profound understanding of the diverse needs of i.e., medical experts, professionals, and laypersons. This enables us to skillfully convey complex data and scientific contexts in a manner that resonates with each stakeholder, ensuring clarity and relevance in our communication.
Regulatory Medical Writing
For the whole range of study documents – from outline/ synopsis and protocol to patient materials as well as study report and scientific publications, we offer a comprehensive suite of medical writing services. Whatever your needs, we have the expertise to deliver:
- Protocol Synopses and their Layperson Summaries
- Clinical Study Protocols and Respective Amendments/ Modifications
- Patient Information and Informed Consent Forms
- Interim Study Reports
- Final Clinical Study Reports
- Summary of Results along with a Layperson Summary of the Study Results
- Study Results Posting in Public Databases
- Documents for German DIGA Fast-Track Procedure
- Protocol for German Application Accompanying Data Collection (Anwendungsbegleitende Datenerhebung, AbD)

Publication Services
Our team excels in crafting scientific manuscripts for publication in peer-reviewed journals. From original research articles to review papers, we ensure that your findings are communicated with clarity, accuracy, and adherence to journal-specific guidelines. We prepare for you:
- Conference Abstracts and Poster Publications
- Manuscripts for Journal Articles
- Marketing Materials
- Training Materials
- Slide Decks for Oral Presentations or Trainings
Specialized Team
Unlike larger agencies, we pride ourself on being a small, closely-knit group of experts. This enables us to maintain a high level of collaboration, ensuring that your project receives the attention it deserves. Our assets are:
- Wealth of experience in clinical research
- Extensive knowledge of a broad range of indications – from A as auto-injector to T as transthyretin familial amyloid polyneuropathy or U as ulcerative colitis, with emphasis on oncologic indications and Medical Devices
- All medical writers are scientists – most of them holding postgraduate qualifications in Life Sciences
- All medical writers participate in workshops at conferences of the European Medical Writers Association (EMWA) to maintain accreditation in the EMWA
- Membership in professional networks (e.g. EMWA, DGPharMed) ensures continuous education and training on relevant guidelines and regulations to ensure high-quality services
- Close collaboration with the AMS clinical operations and biostatistics experts permits our medical writers to give valuable input and support right from the very beginning of a study
Tailored Solutions
We recognize that each project is unique, and our approach reflects this understanding. Whether you require regulatory documents, clinical study reports, manuscripts, or any other medical writing service, we customize our solutions to fit your specific requirements.
Project Management

The role of the Project Manager is key in ensuring the success of any Clinical Study. Throughout your project, our Project Managers at AMS will proactively engage with you and your team, offering innovative solutions to overcome project challenges. Clients frequently endorse the exceptional responsiveness of our Project Managers, a key element in achieving project success. Ultimately, these efforts contribute to the development of robust budgets, high-quality outcomes, and adherence to timelines.
Your dedicated AMS Project Manager will closely collaborate with you and your team, serving as the central point of contact for all project-related tasks. They will furnish a comprehensive plan for project progression and associated timelines, incorporating modern techniques such as EDC-derived Management Reports and collaboration platforms, as well as Clinical Trial Management Systems.
Complementing the Project Manager, the central AMS Clinical Trial Administration team will handle all aspects of project administration, functioning as valuable assistants to the PM-team. Responsibilities include operating Clinical Trial Management Systems (CTMS), setting up and administering the Trial Master File (eTMF), managing the supply chain, offering investigator helplines, and overseeing site payments.
AMS has consistently demonstrated its commitment to timelines, with CRO Benchmarking evaluations consistently affirming that AMS teams have maintained control over 100% of timelines. Partner with AMS for a proactive and efficient project management experience that ensures the success of your Clinical Study.
Partner


Systems
While Clinical Trial Management Systems (CTMS) and Electronic Master File (eTMF) have become the absolute standard, the set-up, implementation, validation and maintenance of these clinical systems involves considerable financial aspects for licenses and capacity investments (e.g. system administrators, validation managers). AMS provides eTMF as a Service to you as an option to have your own eTMF/ CTMS platform fully maintained by AMS thus reducing both your costs and your in-house capacity. Please approach us for more information on the existing options.
Our teams rely on Smenso, an internal robust project management platform to streamline how we plan, execute, and track projects. With Smenso, we effectively organize workflows, monitor progress, and collaborate seamlessly across AMS teams.
Key benefits of using Smenso in our projects:
- Smart Resource Planning: Optimize team workloads and address potential bottlenecks before they occur, ensuring efficient use of resources
- Strategic Planning: Easily map out project timelines, milestones, and goals of all projects to set a clear path to success
- Seamless Collaboration: Facilitate team communication and coordination, keeping everyone aligned and engaged
Partnering with Smenso enables us to lead our projects to success with confidence and precision. By leveraging their innovative tools, we deliver impactful results while maintaining efficiency throughout every phase of the project lifecycle.
Regulatory Affairs
Global regulatory requirements for clinical development are becoming more complex – the AMS Regulatory Affairs team, together with our other services, has the expertise to navigate you through these requirements when planning your clinical development strategy.
Core services include:
- Clinical Trial Applications
- Writing Essential Documents
- Gap Analysis Prior to Submissions
- Meetings and Briefing Packages, Advice Meeting Attendance
Covering:
- Phase I – Including First In Man (FIM) Design
- Phase II – IV
- Non-Interventional
- Medical Device
- Post-Market Clinical Follow-up Studies (PMCF)
- Mandated Safety Studies
- Real World Evidence (RWE)

Data Management

AMS follows the CDISC data standards, for the collection of data according to CDASH and for analysis and submission of data according to ADaM and SDTM.
AMS uses the clinical database management system (CDMS)/ Electronic Data Capture (EDC) system Clincase® and Oracle Clinical One for all types of clinical trials. AMS has a partnership with Oracle in a so called CRO Growth Initiative since 2023. Based on trial needs, AMS will recommend the most suitable EDC system. Medidata Rave® system could also be used, having the eCRF setup/Database build by the vendor (Medidata) itself.
In addition AMS has developed its own electronic Patient Reported Outcome system (AMS-ePRO® ) for the online collection of patient questionnaires and patient diaries allowing direct entry by patients themselves using their own devices (any type of smartphones, computers, tablets, etc.).
For coding with MedDRA and WHO Drug Dictionary AMS uses the PACE® system from Clearight®.
AMS Validation Managers with in-depth know-how in the field of Computer System Validation (CSV) ensure installation and use of AMS fully validated 21 CFR part 11 compliant systems in Data Management.
AMS Data Management (DM) provides all services from eCRF/ database development, data collection and cleaning throught database lock and provision of data for statistical analysis.
Our services include:
- eCRF/ Database Development and Change Management
- Edit Check Programming
- Data Cleaning and Clinical Data Review
- Query & Discrepancy Management
- Coding with WHO-DD and MedDRA Dictionaries
- Handling of External Data (Import, Reconciliation)
- Provision of Reports for Patient Data/ Study Progress Status
- Creation and Maintenance of all DM Documents
- EDC/ ePRO User Administration
- Database Lock Activities
AMS Electronic Patient Reported Outcomes (AMS-ePRO®)
AMS-ePRO® Supports Clinical Research
With AMS-ePRO®, you can now capture your patients’ data anytime and anywhere. AMS-ePRO® helps expedite the collection of clinically relevant data for your trial.
AMS has over 25 years success record of supporting many pharmaceutical, biotechnology and medical device companies in running clinical trials of all phases, indications and complexities. As clinical research evolves, and patient engagement is increasingly important, AMS has developed a fully validated electronic ePRO solution.
AMS-ePRO® is a fully validated electronic solution which not only conforms with GCP and GDPR, but is flexible and more accommodating for the patients as they can utilize their own device.
AMS-ePRO® is widely used in a large number of indications.

Monitoring/ Risk-based Monitoring/ Remote and Centralized Monitoring

AMS stands out as a leader in clinical research, boasting a team of dedicated Clinical Research Associates (CRAs) employed across Europe and expanded by global alliance partners.
Our success is consistently validated by the positive feedback from our clients.
Our CRAs at AMS bring a wealth of experience across various indications, with a particular emphasis on oncology and immunology, among others. Specialized teams within AMS operate with precision in early development clinical trials and late-stage projects, ensuring tailored approaches for optimal outcomes.
Pioneering advancements in monitoring techniques, AMS has been at the forefront of innovation over the past decade. We have seamlessly integrated processes like Remote Monitoring, Centralized Monitoring, and Risk-based Monitoring into our practices, ensuring our monitoring approaches evolve with the industry.
AMS Monitoring is facilitated by a fully validated and user-friendly Clinical Trial Management System (CTMS) with customized workflows for each clinical trial design. Additionally, we offer state-of-the-art ePRO and wearables technologies, unlocking new possibilities in decentralized data collection designs and real-world data acquisition.
Experience the difference with AMS Monitoring – a commitment to excellence backed by a decade of innovation and expertise in advancing clinical research monitoring.
Clinical Safety and Pharmacovigilance
AMS Clinical Safety and Vigilance Experts, with diverse backgrounds in medical, pharmaceutical and natural sciences, combine the expertise in safety monitoring, regulatory compliance, risk assessment, and communication to support you with safety activities throughout multifaceted global and national legislation on medical device and in-vitro diagnostics development journey.
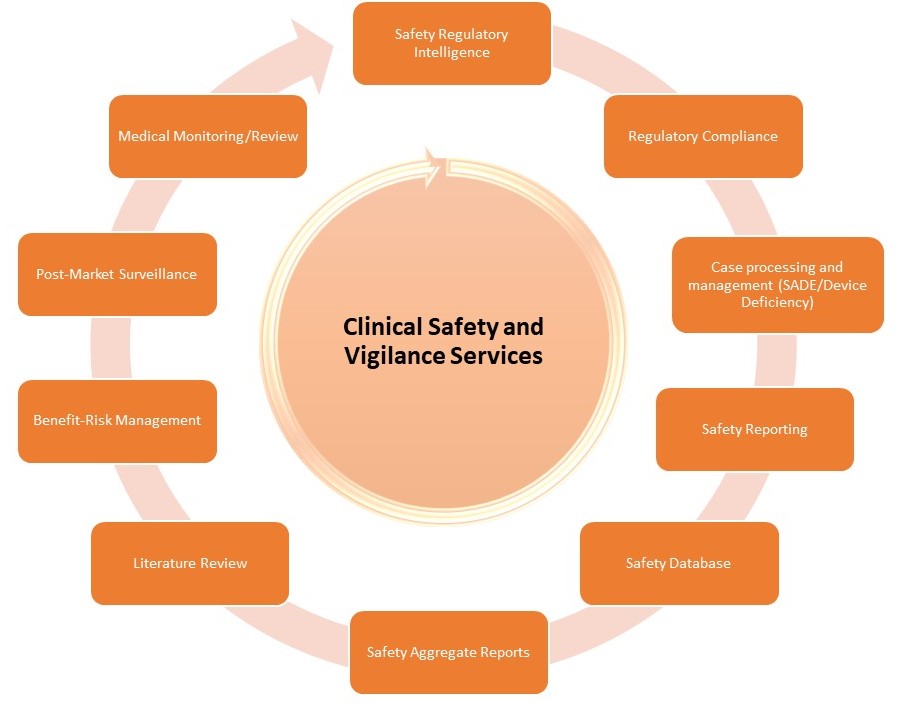
Our team offers you a full range of safety service or custom-made single service packages according to your needs. Our services include:
- Safety Regulatory Intelligence: Gathering, and summarizing information on the complex device guidelines and regulations for each participating country
- Regulatory Compliance: Preparing and/ or delivering safety reporting obligations in a timely manner ensuring compliance to regulatory requirements specific to medical device and IVD, including FDA and EU regulations, ISO standards, and other applicable national laws.
We also facilitate communication channels with other manufactures to report and receive updates on safety concerns - Case Processing and Management (SADE/ Device Deficiency): Including SAEs/ Pregnancies reviewing minimum criteria/ missing information/ plausibility/ discrepancies, conducting medical reviews, assessing anticipation, managing follow-up requests, performing data entry and QC in required databases (including coding activities and narrative writing), preparing standard reporting forms (MDCG reporting, Individual notification Forms as needed), unblinding, tracking cases, and providing listings for reconciliations
- Safety Reporting: submission of cases to competent authorities, ethic committees and investigators to ensure compliance with the required timelines and submission requirements of local and global health authorities
- Safety Database: Including set-up and maintenance
- Safety Aggregate Reports: Preparation and submission of mandatory, high-volume reports including Clinical Study Report (CSR), Annual Safety Report (ASR), or Summary evaluations as needed
- Literature Review
- Benefit-Risk Management
- Post-Market Surveillance: Provide post-market surveillance services to monitor the safety of medical products once they are available to the broader population and management of post-market safety reporting obligations
- Medical Monitoring/ Review: provide highly experienced physicians in all therapeutic areas who are responsible for maintaining the medical oversight in a study in order to minimize the patient’s risks inherent in a clinical study, ensure patient safety to the fullest extent possible continuously throughout the study, and to maximize data integrity through ongoing reviews and interactions with sites and the Sponsor
- They are available for after-hours questions from sites, including 24/7 availability for medical emergencies, to support and guide the study team and ensure patient safety
- They are responsible for providing medical support and ongoing guidance to the clinical trial team, including monitors and investigator sites, for protocol-related issues (such as subject eligibility and protocol interpretation)
- They assess and respond to site questions/issues (including inclusion/exclusion criteria, clinical and safety-related trial questions, such as those related to the Investigational Medicinal Device (IMD) or in-vitro diagnostics (IVD), serious adverse events (SAEs) or serious adverse device effects (SADEs) including Device Deficiencies (DD) processing, concomitant medication, or medically-related queries from sites
Because our expertise in clinical safety and vigilance services is tailored to the medical device field, you will be easily navigated through Interventional Clinical Investigations of all developmental stages, Non-Interventional Studies (NIS), and Post Marketing Clinical Follow-ups (PMCF).
Take advantage of AMS competency and reliability.
Biostatistics
All services required in the lifecycle of clinical development projects, beginning with biostatistical consulting during development of the project concept up to contributions for submissions or HTA dossiers and post approval studies are provided.
AMS services include:
- Biostatistical consulting and writing for clinical trials and observational research studies
- Sample size calculations
- Randomization (with optional integration of the randomization list into the eCRF)
- Support of/ participation in Monitoring Committees
- Biostatistical review and contributions to study protocols/ study reports/ posters/ manuscripts
- Comprehensive and detailed statistical analysis plans (SAP)
- SAS Programming for the statistical analysis, for review listings, periodic safety reports or trial results reporting to EudraCT database
- Regulatory submission suitable implementation of CDISC SDTM/ ADaM standards
- Health Economics, Patient surveys, Burden of Disease studies, cost-effectiveness analyses, quality of life evaluation
- Specific knowledge in innovative clinical trial designs like adaptive designs
- Continuous training on all applicable guidelines is part of our Quality Management System
All services are performed with validated systems (SAS, nQuery, Pinnacle 21 Community Validator) following all applicable guidelines (e.g., GCP, ICH E3/E9, 21 CFR part 11).
Use AMS experience to your benefit and let us help you to select the optimal study design and appropriate endpoints for the objectives of your project. We provide time- and cost-efficient conduct of all services with high quality deliverables.
Within a project, the Biostatisticians provide ongoing support and consultation to the Sponsor and the project team. Biostatistics liaises closely with Sponsor scientists, project managers, data managers and medical monitors to provide the optimal solution for your needs.
References







