Biostatistics
Since the introduction of the AMNOG in 2011 in Germany, we provide our clients with comprehensive advice and support on all biostatistical issues concerning German benefit dossiers. We develop strategies to meet the requirements of IQWiG and the G-BA and at the same time achieve the best possible result for your product.
Over 25 biostatisticians support you in all statistical aspects during the entire benefit assessment process.
Our Services
- Methodology
- direct and indirect comparisons (adjusted/non-adjusted, historical and external control groups)
- (Network) meta-analyses
- Propensity score matching
- Matching-adjusted indirect comparisons (MAIC)
- Surrogate validations
- Survival time analyses
- Multiple regression
- ...and other methods. Feel free to contact us! - Additional Analyses
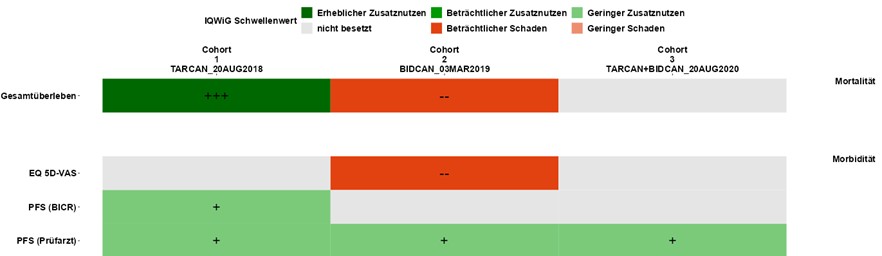
For your benefit dossier, we carry out all additionally required statistical analyses according to the current requirements of IQWiG and G-BA and support you in communicating with your global colleagues. - Data Visualization (ShinyViz)
Fast visualization and individualized graphical presentation of analysis results to determine the best possible dossier strategy.
- European HTA
At AMS, we have been active in the field of EU HTA since 2015. Among other things, we support you in statistical evaluations around JSCs, rapid REAs and JCAs. - Routine practice data collection (anwendungsbegleitende Datenerhebung, AbD)
As pioneers in the field of routine practice data collection (anwendungsbegleitende Datenerhebung, AbD), we accompany you at every step through this recently established process. We offer strategic consultation, individually tailored workshops, active participation during the stakeholder participation process and preparation of study documents including study protocols and SAP.



