AMS electronic Patient Reported Outcomes (AMS-ePRO®)
AMS-ePRO® supports Clinical Research
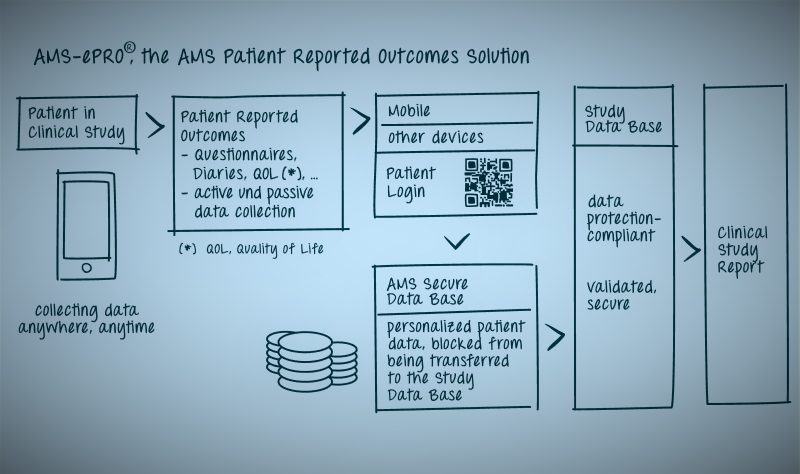
You can now capture your patients data Anytime Anywhere with AMS-ePRO® to provide you with more clinically relevant data to help your trial move.
AMS has a 25 year success record of supporting many pharmaceutical, biotechnology and medical device companies in running clinical trials of all phases, indications and complexities. As clinical research evolves, and patient engagement is increasingly important, AMS has developed a fully validated electronic ePRO solution.
AMS-ePRO® is a fully validated electronic solution which not only conforms with GCP and GDPR, but is flexible and more accommodating for the patients as they can utilize their own device. AMS-ePRO® is widely used in a large number of indications.
AMS-ePRO® - flexible solutions
Collect what you want! Anywhere Anytime
Collect Patient Reported Outcomes:
- Patient diaries: simple or complex
- Quality of life or health related quality of life data. QOL, HQRL
- Surveys
- Geofencing, Call-tracking, scheduler, reminders.
Bring Your own Device – Concept
Web-based Data Collection with patient own devices (e.g. smartphones, tablets or computers)
System fully validated
according to international computer system validation
standards (21 CFR part 11 and Annex 11)
System GDPR Compliant
in accordance with the Data Protection requirements (GDPR)
Reporting
The collected data in AMS-ePRO® could be exported or reported based on client wishes.