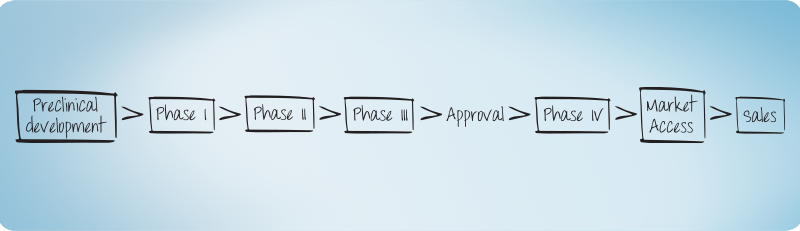
Clinical Development - read more
Phase I, IIa, IIb/IIIa, IIIb -
A dedicated and proven team will provide:
- Protocol development
- Clinical Trial Feasibility
- Indication experience
- Coverage
- Central Study management
- European central submission services
- Risk Management Plans
- On-site Monitoring and centralized Monitoring techniques as well as Risk-based Monitoring approaches
- Reporting
- Quick study set-up
- Innovative patient recruitment support solutions