Routine practice data collection (anwendungsbegleitende Datenerhebung, AbD)
We bring extensive firsthand experience from actively engaging in multiple AbD procedures. Our services include strategic consultation, individually tailored workshops, active participation during the stakeholder participation process and preparation of study documents including study protocols and SAP. Our interdisciplinary teams are specialized in biostatistics, medical writing, project management, and clinical operations.
With our hands-on experience and multidisciplinary approach, we provide comprehensive support and guidance throughout your entire AbD process.
Our services
- Setup
We support you in the strategic planning of the AbD process by offering consultation on budget planning and stakeholder involvement. - Workshops
In individually tailored workshops, we support you to develop the course of action, conduct PICO-Scoping and analyze potential gaps (GAP-Analysis). - Engagement with G-BA and IQWiG
Our experienced consultants proactively guide you during the participation process and support you in creating written statements in response to the IQWiG AbD concept as well as during participation in the stakeholder process. - Study documents
You benefit from our HTA- and Clinical Operations-experts´ extensive experience in writing and revising study documents and SAP. - Methodological and strategic consultation
We share our experience and know-how regarding the challenging methodological aspects of the AbD, including calculation of patient numbers, systematic confounder identification, recording of patient reported outcomes and collaboration with registries. - Implementation of the AbD
Our team also supports you after the start of the routine practice data collection. We generate status reports, interim analyses, and final evaluations and interpretation.
AbD in early benefit assessment
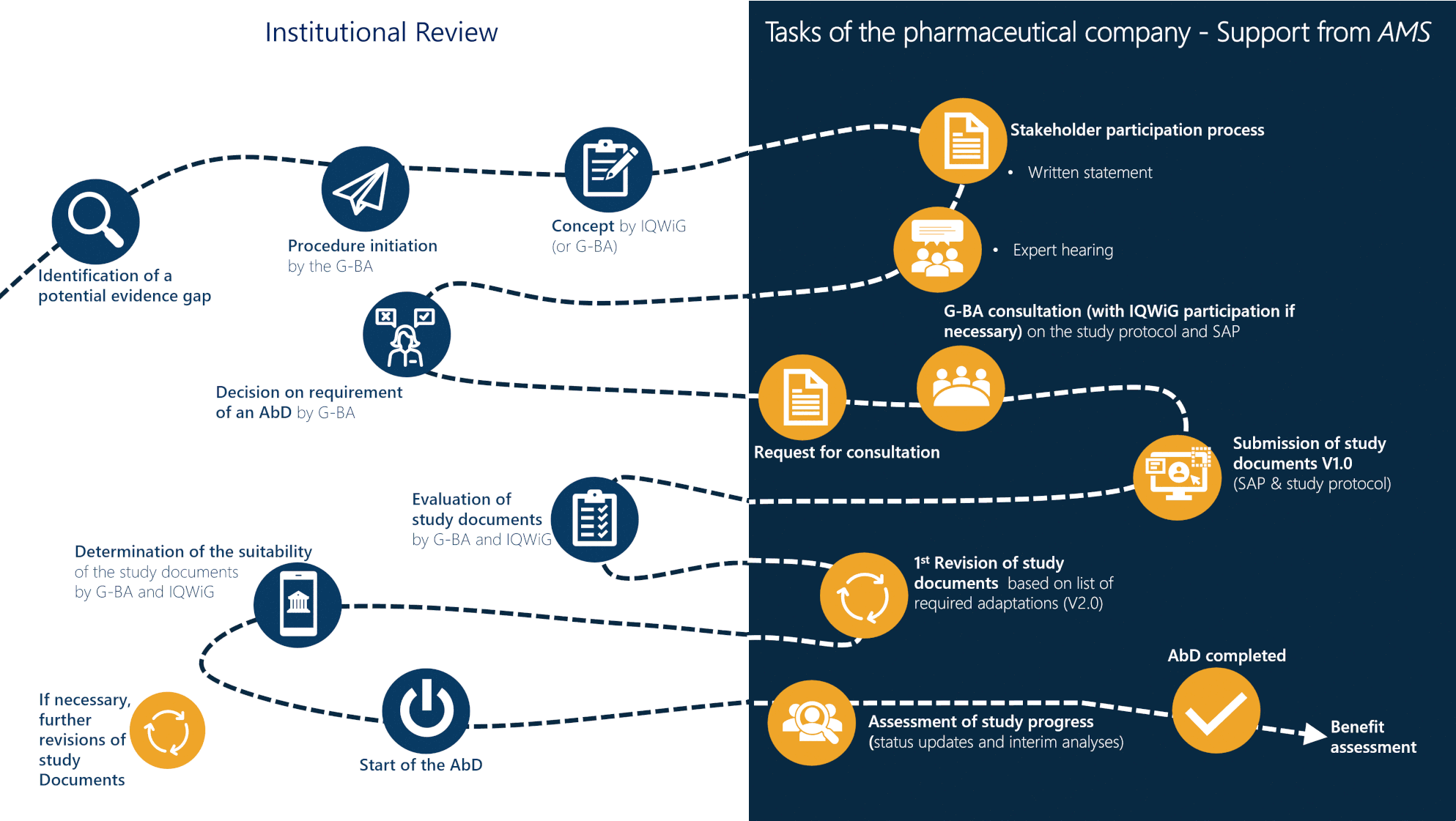
Since 2020 the G-BA can demand a post-launch routine practice data collection (anwendungsbegleitende Datenerhebung, AbD) for new pharmaceutical products with certain approval pathways (conditional approval or approval under exceptional circumstances) or Orphan Drugs. The AbD process is initiated when available evidence for a benefit assessment is insufficient.
AbD course of action: When does what happen?
The AbD process is described in chapter 5 of the G-BA procedural rules (Verfahrensordnung) and encompasses several steps. The development of the AbD concept by IQWiG and the written statement of the pharmaceutical company are essential steps in the process. If an AbD is demanded, the pharmaceutical company is required to submit study documents (SAP and study protocol), which describe the methodology of the AbD. After the completion of the AbD, a new benefit assessment of the pharmaceutical product is conducted.